Current Research
Section II. The initiation of differentiation from bloodstream to procyclic forms.
When in the bloodstream, stumpy cells are irreversibly committed to differentiate to procyclic forms, which happens only once they are taken up into the tsetse fly. To investigate the controls underlying this step, we have focused on a phosphotyrosine phosphatase activity implicated in differentiation control. In particular, we have identified a PTP (TbPTP1) which, when inactivated either genetically or pharmacologically stimulates spontaneous differentiation of bloodstream forms to procyclic forms. Importantly we saw that this only occurred in a small subset of cells. We believed that these were cells that had committed to form stumpy cells. This was subsequently confirmed by demonstrating that uniform populations of stumpy cells differentiated synchronously, and efficiently in response to a cell permeable PTP1B inhibitor.
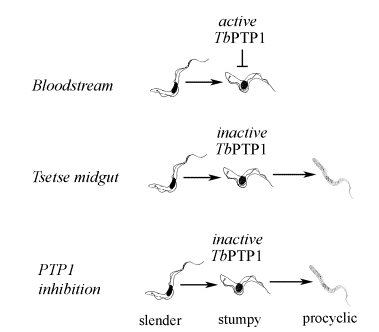
This suggested a new model for the control of trypanosome differentiation. In this model, bloodstream parasites commit to stumpy formation and become competent (stumpy*) to differentiate to procyclic forms. However, they are held arrested in this state in the bloodstream by the action of TbPTP1. Then, upon uptake by the tsetse fly, this inhibition is removed, and the parasites progress unhindered to procyclic forms. This model has two important implications. Firstly, it supports the concept that proliferative slender cells are not competent to differentiate to procyclic forms unless they commit to the early events of stumpy formation, in particular cell-cycle arrest. Also it suggests that the default pathway for stumpy forms is to differentiate directly to procyclic forms, and that this differentiation is inhibited in the bloodstream by the action of TbPTP1. In other words, differentiation primarily results from release of the “brake” rather than application of the “accelerator”.
We are now interested in the function of this molecule in terms of its substrates, and interactions with other cellular proteins that define the cell cycle arrest characteristic of stumpy forms. Using a "substrate-trapping" mutation of TbPTP1, we selected its cellular protein target, which was a second phosphatase, TbPIP39. Interestingly, TbPIP39 activates TbPTP1, which represses its own activity- a negative feedback loop that is broken by the differentiation triggers citrate and cis-aconitate. TbPIP39 is ultimately delivered to a specialised set of organelles in the parasite called glycosomes. These compartmentalise the parasite's glycolytic reactions, as well as being involved in some other biochemical functions. By identifying the targets for TbPIP39 within the glycosome we aim to understand how trypanosome signalling is linked to parasite metabolism.
For more information on this theme, see:
- Szöőr, B; Wilson, J., McElhinney, H.; Tabernero, L. and Matthews KR (2006)
Protein tyrosine phosphatase TbPTP1: a molecular switch controlling life cycle differentiation in Trypanosoma brucei. Journal of Cell Biology 175(2):293-303.
Szöor B, Ruberto I, Burchmore R, Matthews KR. (2010)
A novel phosphatase cascade regulates differentiation in Trypanosoma brucei via a glycosomal signaling pathway.
Genes and Development. 15;24(12):1306-16.
Balázs Szöõr , Naomi Dyer, Irene Ruberto, Alvaro Acosta- Serrano and Keith R. Matthews (2013)
Independent pathways can transduce the life-cycle differentiation signal in Trypanosoma brucei
PLoS Pathogens 10.1371/journal.ppat.1003689

